Understanding the Differences Between Botulinum Toxin Type A Brands in Australia
Comparing Botulinum Toxin Type A Products in Australia: A Clinically-Focused Overview
This article is for educational purposes only. It is non-promotional and based on published evidence and current regulatory approvals in Australia. It does not endorse any specific brand.
As a doctor who has been injecting botulinum toxins for nearly two decades, I’ve used a wide range of products available in Australia. It’s often puzzled me how similar they can appear on the surface — yet how differently they may behave. We rarely have the opportunity to compare them directly in a controlled setting, and conducting split-face or head-to-head trials in routine practice is neither practical nor ethical.
Instead, we must turn to:
-
Scientific and regulatory data,
-
Differences in formulation and manufacturing, and
-
Independent clinical trials where available.
This article offers a detailed, evidence-based comparison of the major botulinum toxin A formulations currently approved in Australia — exploring what’s in the vial, how they differ, and what the data tells us about their performance.
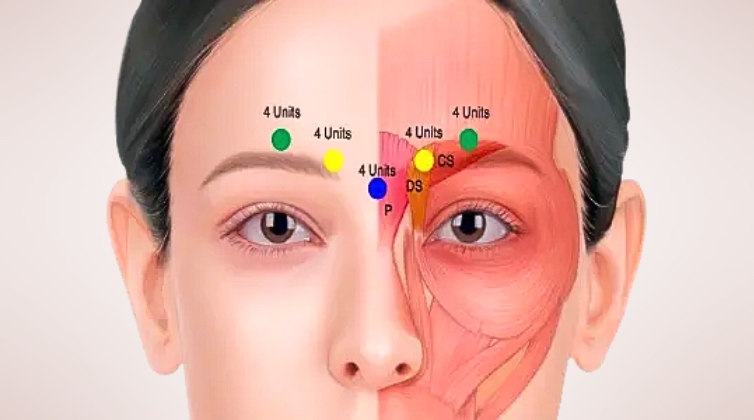
1. Available Botulinum Toxin A Products in Australia
The following botulinum toxin A products are approved for aesthetic use in Australia:
| Brand | Active Ingredient | Sponsor in Australia |
|---|---|---|
| Botox® | onabotulinumtoxinA | Allergan (AbbVie) |
| Dysport® | abobotulinumtoxinA | Ipsen |
| Xeomin® | incobotulinumtoxinA | Merz |
| Nuceiva® | prabotulinumtoxinA | Evolus |
| Relfydess® | relabotulinumtoxinA | Galderma |
| Letybo® | letibotulinumtoxinA | Croma Australia Pty Ltd |
2. What’s in the Bottle? Neurotoxin Structure and Protein Load
All of these products share the same 150 kDa core botulinum neurotoxin, which blocks acetylcholine release at the neuromuscular junction. However, the formulation and protein content differ:
| Product | Complexing Proteins | Final Form | Notes |
|---|---|---|---|
| Botox | Yes | Powder | Lyophilised; includes accessory proteins |
| Dysport | Yes | Powder | Larger protein complex; broader diffusion profile |
| Xeomin | No | Powder | “Naked” toxin; vacuum-dried, only 150 kDa neurotoxin |
| Nuceiva | Yes | Powder | Vacuum-dried; proprietary purification to reduce inactive proteins |
| Relfydess | No | Liquid | Ready-to-use; PERL™ technology; lacks complexing proteins |
| Letybo | Yes | Powder | Freeze-dried; contains accessory proteins; limited Western data |
Complexing proteins serve no functional role once injected but may impact immunogenicity and diffusion (Frevert, Dressler 2010). In nature, they help the neurotoxin survive digestion, but they’re unnecessary for intramuscular use. Products like Xeomin and Relfydess omit them to reduce the risk of antibody formation.
3. Excipients and Diluents: Supporting Ingredients and Injection Considerations
Excipients (inside the vial)
Excipients are inactive stabilising agents that support the structure and viability of the botulinum toxin protein. These vary slightly between products:
| Product | Key Excipients/Stabilizers | Notes |
|---|---|---|
| Botox® | Human serum albumin (HSA), NaCl | Classic formulation; widely used |
| Dysport® | HSA, lactose | Contains lactose — relevant for dairy-allergic patients |
| Xeomin® | HSA, sucrose | Sucrose supports stability in vacuum-dried formulation |
| Nuceiva® | HSA, NaCl | Similar profile to Botox |
| Relfydess® | HSA, NaCl | Liquid format; PERL™ tech; no reconstitution needed |
| Letybo® | HSA, NaCl | Lyophilised format; same excipients as Botox |
Human serum albumin (HSA) is used to prevent protein aggregation and maintain toxin stability during storage and transport.
Diluents: Manufacturer Recommendations and Clinical Practice
Most products are reconstituted using preservative-free saline (0.9% NaCl). Some clinicians prefer preserved saline (with benzyl alcohol) to reduce pain on injection, but this is only suitable for powdered toxins.
Diluents: Manufacturer Recommendations and Clinical Practice
| Product | Requires Reconstitution? | Manufacturer-Recommended Diluent | Preserved Saline Supported? | Clinical Notes |
|---|---|---|---|---|
| Botox® | Yes | Preservative-free 0.9% NaCl (USP) | ❌ Not supported | Some clinicians use preserved saline off-label to reduce sting. |
| Dysport® | Yes | Preservative-free 0.9% NaCl (USP) | ❌ Not supported | Large-vial format (300–500U); dilution varies by indication. |
| Xeomin® | Yes | Preservative-free 0.9% NaCl (USP) | ❌ Not supported | Vacuum-dried; minimal excipients; precise reconstitution preferred. |
| Letybo® | Yes | Preservative-free 0.9% NaCl (USP) | ❌ Not supported | Use within 24 hrs after reconstitution; 50U or 100U vials. |
| Relfydess® | ❌ No (liquid format) | Not applicable | ❌ Not applicable | Ready-to-use; cannot be diluted or mixed with preserved saline. |
While preserved saline (with benzyl alcohol) may reduce sting, this use is off-label for all powdered products and not supported by any manufacturer’s product information. Relfydess, being a liquid, must be used as supplied without modification.
4. Pain on Injection: Factors That Influence Patient Comfort
The discomfort associated with botulinum toxin injections can vary between products and is influenced by several factors, including:
-
Diluent composition (e.g. preserved vs preservative-free saline)
-
Final pH after reconstitution
-
Formulation format (powder vs liquid)
-
Volume and concentration injected
-
Injection technique and needle gauge
💉 Preserved vs Preservative-Free Saline
Most products are reconstituted with preservative-free 0.9% sodium chloride, as per manufacturer instructions. However, some clinicians choose to use preserved saline (containing 0.9% benzyl alcohol) off-label to reduce injection discomfort.
While preserved saline may slightly reduce the pH of the reconstituted solution, its primary benefit is that benzyl alcohol acts as a local anaesthetic, often making injections feel less painful — particularly in sensitive areas like the glabella or lips.
This approach is off-label and should be considered in the context of product stability and patient safety.
Relfydess®, as a ready-to-use liquid, cannot be diluted or reconstituted, so there is no opportunity to modify its injection characteristics with saline.
⚗️ pH Differences
The pH of the final solution may influence injection sting. Lower (more acidic) pH can cause more discomfort upon injection. Approximate pH values below reflect reconstitution with preservative-free saline, as per manufacturer specifications.
| Product | Approx. pH after reconstitution | Pain on Injection |
|---|---|---|
| Botox® | ~7.0 | Low (with preserved saline); mild otherwise |
| Dysport® | ~7.0 | Mild to moderate |
| Xeomin® | ~7.3–7.4 | Generally mild |
| Nuceiva® | ~7.0 | Mild |
| Relfydess® | ~5.5–6.0 (liquid, pre-buffered) | Anecdotally more painful |
| Letybo® | ~7.0 | Mild |
While most reconstituted products are near physiologic pH, Relfydess® appears to be slightly more acidic, which may contribute to increased injection discomfort. Some clinicians have proposed buffering acidic formulations with sodium bicarbonate, but no published studies support this approach for Relfydess.
The ideal pH for least sting during injection is close to physiological pH — approximately 7.3 to 7.4.
⚠️ Clinical note: Buffering with sodium bicarbonate is an off-label technique and not recommended unless supported by product-specific stability data.
5. Onset and Duration of Effect
All botulinum toxin A products act via the same mechanism: blocking acetylcholine release at the neuromuscular junction. However, subtle differences in formulation and dose may influence how quickly the effect begins and how long it lasts.
| Product | Reported Onset | Comments |
|---|---|---|
| Relfydess® | Day 1 | In clinical trials, 39% of patients reported improvements in frown lines and 34% in crow’s feet within 24 hours. Median onset is 2–3 days. |
| Dysport® | 2–3 days | Often noted for faster onset; possibly due to wider diffusion and different protein complex. |
| Xeomin® | 3–4 days | Clinical experience suggests onset is comparable to Botox; some reports suggest slightly quicker onset. |
| Botox® | 3–5 days | Initial effects typically appear within 3–5 days; full results may take up to 10–14 days. |
| Nuceiva® | 3–4 days | Similar to Botox in clinical trials. |
| Letybo® | 2–3 days (estimated) | Limited published data in Western use; some reports suggest faster onset. |
📌 Clinical note: While Relfydess® shows a rapid onset in a subset of patients, individual responses can vary. Differences of 1–2 days in onset are often clinically insignificant unless rapid onset is a priority (e.g., pre-event treatments).
6. Immunogenicity: Reducing the Risk of Antibody Formation
While botulinum toxin A is generally well-tolerated, repeated treatments may, in rare cases, lead to the development of neutralizing antibodies (nAbs). These antibodies bind to the botulinum toxin and reduce or eliminate its clinical effect, resulting in primary or secondary non-response.
This is of particular concern in aesthetic practice for:
-
High-frequency users (e.g., every 3 months),
-
High-dose areas (masseters, platysma),
-
Patients with therapeutic indications using higher cumulative doses.
🧬 What Contributes to Immunogenicity?
Immunogenicity is most strongly linked to:
-
Total protein load: Includes both active and inactive components.
-
Presence of non-therapeutic proteins: Such as complexing proteins, bacterial DNA fragments, and other impurities.
-
Formulation and purification methods: Cleaner, purer formulations with fewer bacterial remnants are less likely to provoke an immune response.
🧪 Comparative Risk: Formulation and Immunogenicity
| Product | Complexing Proteins | Purification Method | Relative Immunogenicity Risk | Notes |
|---|---|---|---|---|
| Xeomin® | ❌ No | Two-step chromatography | 🔽 Lowest known | “Naked” toxin; only active 150 kDa neurotoxin (Frevert, 2009) |
| Relfydess® | ❌ No | PERL™ technology (proprietary) | 🔽 Presumed low | Liquid formulation; no complexing proteins; limited long-term data |
| Nuceiva® | ✅ Yes | Vacuum-dried; reduced inactive toxin | ⚖️ Moderate–Low | Cleaner than Botox, but retains complexing proteins (Goldman, 2019) |
| Botox® | ✅ Yes | Standard purification | ⚠️ Moderate | Long clinical history; small risk of antibody formation in high-dose users |
| Dysport® | ✅ Yes | Larger protein complex | ⚠️ Moderate–High | Larger total protein load may increase risk |
| Letybo® | ✅ Yes | Limited published detail | ❓ Unknown | Insufficient Western clinical data; presumed moderate risk |
📘 Supporting Literature
-
Frevert, 2009: Demonstrated that accessory proteins are not necessary for clinical effect and may contribute to immunogenicity.
-
Pickett, 2011: Advocated for purified neurotoxins in repeat-use aesthetic patients to reduce the likelihood of nAbs.
-
Goldman et al., 2019 (EV-001 & EV-002): Showed non-inferiority of Nuceiva to Botox with no increase in adverse immune responses, supporting the safety of its purification method.
📌 Clinical Considerations
-
Avoid overtreatment: Reinjection within 3 months may elevate immunogenicity risk. Spacing treatments 12+ weeks apart is generally recommended.
-
Use the lowest effective dose: This reduces cumulative protein exposure over time.
-
Rotating injection sites is not protective — antibody production is systemic, not site-specific.
-
Once neutralising antibodies develop, patients may experience non-responsiveness to all botulinum toxin A products, as the immune response targets the shared core neurotoxin, not the accessory proteins.
🔍 For long-term users or patients requiring high doses, choosing low-immunogenicity products like Xeomin or Relfydess may help maintain treatment efficacy over time.
❗ While all current formulations are considered safe, the lowest immunogenicity profile remains with Xeomin, due to its exclusive content of the 150 kDa neurotoxin and lack of complexing proteins and long-term data.
7. Dosing Equivalence and Non-Inferiority Studies
Botulinum toxin units are not interchangeable across brands. Each manufacturer defines “one unit” based on its own LD50 bioassay (typically mouse-based), which is product-specific. Therefore, unit-to-unit comparisons can only be made based on clinical equivalence studies — often via non-inferiority trials.
📊 Evidence-Based Dosing Conversion Table
| Comparator Toxins | Conversion Ratio (Approx.) | Key Findings | Reference |
|---|---|---|---|
| Botox vs Xeomin | 1:1 | Clinically equivalent in efficacy and safety for glabellar lines and blepharospasm. | Sattler et al., 2010; Roggenkämper et al., 2006 |
| Botox vs Nuceiva | 1:1 | Two Phase III RCTs (EV-001 and EV-002) demonstrated statistical non-inferiority in glabellar treatment. | Goldman et al., 2019 |
| Botox vs Dysport | 1:2.5 to 1:3 | Dysport requires a higher unit dose for equivalent effect; wider diffusion noted. | Trindade de Almeida, 2007; Nestor et al., 2010 |
| Botox vs Letybo | 1:1 (presumed) | Limited direct comparisons; presumed equivalent based on internal studies and use in Asia. | Not yet peer-reviewed |
| Botox vs Relfydess | 1:1 (labelled); dosing varies | Clinical trials suggest 50 units for glabella; duration claims up to 6 months may reflect higher dosing. | Galderma internal documents; no independent published trials as of 2025 |
🔬 Clinical Trial Highlights
Xeomin (incobotulinumtoxinA)
-
Roggenkämper et al., 2006: In blepharospasm patients, Xeomin was non-inferior to Botox at 1:1 dosing, with no significant difference in efficacy or safety.
-
Sattler et al., 2010: Showed equivalent efficacy for glabellar lines using 20 units in a double-blind randomized study.
Nuceiva (prabotulinumtoxinA)
-
EV-001 & EV-002 (Goldman et al., 2019): Multicentre double-blind Phase III trials comparing Nuceiva to Botox in 633 patients. Both trials demonstrated non-inferiority in treating moderate-to-severe glabellar lines at 20 units.
Dysport (abobotulinumtoxinA)
-
Trindade de Almeida, 2007: Confirmed the 2.5:1 conversion ratio with Botox in glabellar line treatment.
-
Nestor et al., 2010: Similar efficacy and duration using 50 units Dysport vs. 20 units Botox for glabella.
💡 Considerations for Relfydess
Relfydess recommends 50 units for glabellar lines, implying that a higher dose is required or preferred for longer duration. This dosing is not yet supported by independent non-inferiority trials, and the 1:1 assumption remains label-based rather than evidence-based.
🧪 Clinical insight: Higher doses of any botulinum toxin are likely to increase duration of effect (Hexsel et al., 2020), but this must be balanced against increased cost, diffusion, and possible immunogenicity.
📌 Clinical Summary
-
1:1 dosing is supported for Botox, Xeomin, and Nuceiva.
-
Dysport requires 2.5–3 times more units to achieve comparable effect.
-
Relfydess may require higher dosing, but this could reflect an intentional strategy to boost duration, not true 1:1 potency.
-
Always follow brand-specific guidelines, especially when switching products.
8. Diffusion and Precision
One of the most clinically significant differences between botulinum toxin products is their pattern of diffusion — how far the toxin spreads from the injection site — which can affect both efficacy and safety.
🌀 What Affects Diffusion?
Botulinum toxin diffusion depends on:
-
Dose and dilution volume
-
Needle gauge and depth of injection
-
Muscle anatomy
-
Formulation properties (e.g., protein load, molecular size, excipients)
While all toxins use the same 150 kDa core neurotoxin, the surrounding proteins, stabilizers, and manufacturing processes may influence spread in tissue.
| Product | Relative Diffusion | Clinical Notes |
|---|---|---|
| Dysport® | Broadest | May diffuse over a wider area; useful in large muscles (masseters, platysma) |
| Botox® | Moderate | Well-characterized; suitable for most facial indications |
| Nuceiva® | Moderate | Claims “precision” due to complexing proteins; no strong diffusion studies published |
| Xeomin® | Precise | “Naked” toxin; some studies and experience suggest tighter spread |
| Relfydess® | Targeted (claimed) | Liquid formulation may reduce post-reconstitution variability; early data only |
| Letybo® | Moderate (estimated) | Limited independent evidence |
📗 Clinical Studies and Expert Opinion
-
Ranoux et al., 2002: Dysport demonstrated greater diffusion than Botox in a forearm model, requiring more precise placement in small muscles.
-
Alam et al., 2008: Suggested Xeomin has similar diffusion to Botox when used at equal doses.
-
Park et al., 2019: Dysport may produce a broader zone of action compared to incobotulinumtoxinA and onabotulinumtoxinA.
🔍 Note: No large, head-to-head diffusion trials exist for Nuceiva, Relfydess, or Letybo as of 2025.
🎯 Practical Considerations (Revised)
-
Use broader-diffusing products (e.g., Dysport) for:
-
Masseters (jawline slimming)
-
Platysma (neck bands)
-
Frontalis (forehead) – when diffuse lift is acceptable
-
-
Use precision-focused products (e.g., Xeomin, Botox) for:
-
Glabella – to minimise risk of spread to the levator palpebrae, which can cause eyelid ptosis
-
Perioral lines (lip lines) – to avoid affecting smile symmetry or speech
-
DAO (depressor anguli oris) – to prevent unintended spread to depressor labii or zygomaticus, which could cause an asymmetric smile or unnatural mouth movement
-
Brow lift (superior frontalis targeting) – where spread could reduce efficacy or produce asymmetry
-
💡 Clinical Insight: Tighter diffusion means greater control — essential in small, functionally significant muscles where even a few millimetres of unintended spread can lead to noticeable side effects.
9. Duration of Effect and Longevity
All botulinum toxin type A products offer a temporary reduction in muscle activity, typically lasting 3 to 4 months. However, small variations in onset, peak effect, and longevity may occur based on product formulation, dose, and individual response.
⏱️ Onset and Duration Comparison
| Product | Onset of Action | Typical Duration | Notes |
|---|---|---|---|
| Botox® | 3–5 days | 3–4 months | Well-characterised timeline |
| Dysport® | 2–3 days | 3–4 months | Often noted for faster onset |
| Xeomin® | 2–4 days | 3–4 months | Anecdotally faster than Botox; may reach full effect more gradually |
| Nuceiva® | 3–4 days | 3–4 months | Similar onset and duration to Botox in clinical trials (EV-001/002) |
| Relfydess® | 1–2 days (claimed) | Up to 6 months (claimed at 50U) | Early onset and prolonged duration claimed by manufacturer |
| Letybo® | 3–5 days (est.) | 3–4 months (est.) | Limited published Western data |
🧪 Note: Onset is defined as the time to first visible effect; duration refers to the time until return of full muscle activity.
🧬 Dose-Response Relationship
A growing body of literature supports the idea that higher doses may lead to longer duration of effect, provided that immunogenicity is not a limiting factor.
-
Hexsel et al., 2020: Demonstrated dose-dependent duration of effect across multiple facial areas using onabotulinumtoxinA.
-
Kane et al., 2010: Higher doses of Botox for glabella extended duration to 5–6 months without additional safety risks.
This is relevant for Relfydess, which recommends 50 units for glabella, a higher dose than the typical 20 units for Botox. This likely contributes to the claimed 6-month longevity, rather than intrinsic formulation differences alone.
⚠️ Clinical takeaway: When comparing product longevity, consider actual dose used — not just brand-specific duration claims.
📝 Summary
-
Most products last 3–4 months with standard dosing.
-
Dysport and Relfydess may act faster, while Relfydess may last longer when used at recommended doses.
-
Dose, dilution, muscle size, and patient-specific metabolism all influence duration.
10. Cost Considerations: Value vs Clinical Utility
While precise pricing cannot be published due to AHPRA advertising guidelines, it’s important to consider cost-effectiveness in relation to clinical outcomes.
💰 Key Factors That Influence Cost Per Treatment:
-
Cost per unit from the distributor (variable across clinics and contracts)
-
Units required for equivalent effect (e.g. Dysport requires ~2.5–3 units for every 1 Botox unit)
-
Duration of effect, which determines reinjection frequency
-
Formulation characteristics (e.g. liquid vs powder, ease of reconstitution, waste minimisation)
-
Wastage potential, especially with liquid products like Relfydess that must be used once opened
🧠 Clinical Insight:
A lower price per unit does not always translate to lower treatment cost if higher dosing is required or results are shorter-lived. In contrast, some higher-cost products may offer better precision or longevity in certain patients, which may balance out over time.
⚖️ Real-World Example:
-
Relfydess suggests 50 units for glabellar lines (vs 20 U for Botox), potentially raising the per-session cost — but this is intended to extend duration to up to 6 months.
-
Dysport offers lower per-unit pricing but requires more units. It may be more cost-effective in large areas (e.g. platysma, masseter).
-
Xeomin, with reduced immunogenicity and good efficacy, may provide value in long-term, repeat users.
🔍 The true measure of value lies in clinical outcomes, not unit pricing alone.
11.Clinical Takeaways and Summary Table
Despite sharing the same core neurotoxin, botulinum toxin A brands differ significantly in formulation, protein structure, diffusion, and clinical use. Selecting the right product depends on practitioner goals, anatomical area, and patient preferences.
🧠 Key Takeaways:
-
Use precise products (e.g. Xeomin, Botox) in areas like the glabella, DAO, peri-oral lines, and brow lifts to minimise risk of spread to nearby muscles.
-
Use broader diffusion products (e.g. Dysport) in larger areas like frontalis, platysma, or masseters.
-
Consider long-term antibody risk in repeat users — naked toxins like Xeomin and Relfydess may carry lower immunogenicity.
-
Formulation differences matter — especially for pain on injection, longevity, and patient tolerability.
-
Duration is dose-dependent — higher doses can prolong effect, as long as immunogenicity is not limiting.
-
There is no universally superior product — matching the formulation to the indication and patient is key.
🧾 Product Summary Table
🧾 Product Summary Table
| Brand | Drying Method | Complexing Proteins | Presentation | Diffusion | Onset | Duration | Immunogenicity Risk | Notes |
|---|---|---|---|---|---|---|---|---|
| Botox | Freeze-dried | Yes | 100 U powder | Moderate | ~3–5 days | 3–4 months | Moderate | Most well-known, original toxin |
| Dysport | Freeze-dried | Yes | 300/500 U powder | Broad | ~2–3 days | 3–4 months | Higher | Useful for large muscle groups |
| Xeomin | Vacuum-dried | No | 100 U powder | Precise | ~2–4 days | 3–4 months | Lowest | “Naked” toxin; well-purified |
| Nuceiva | Vacuum-dried | Yes (purified) | 100 U powder | Moderate | ~3–4 days | 3–4 months | Lower (claimed) | Aesthetic use only. Hi-Pure™ technology. |
| Relfydess | Liquid (ready-to-use) | No | 100 U liquid | Moderate | ~1–2 days | Up to 6 mo* | Low | More painful injection; PERL™ tech |
| Letybo | Freeze-dried | Yes | 100 U powder | Moderate | ~3–5 days | 3–4 months | Unknown | Limited Western data |
*Note: Relfydess claims longer duration based on higher recommended dosing (e.g. 50 U for glabella), not necessarily molecular longevity.